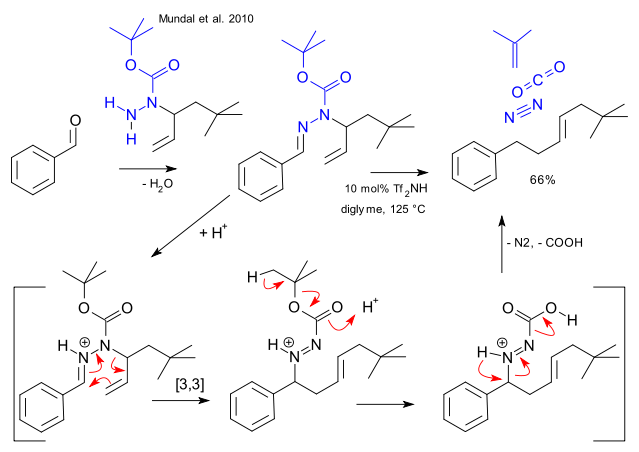
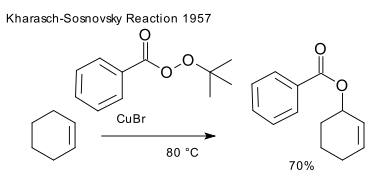
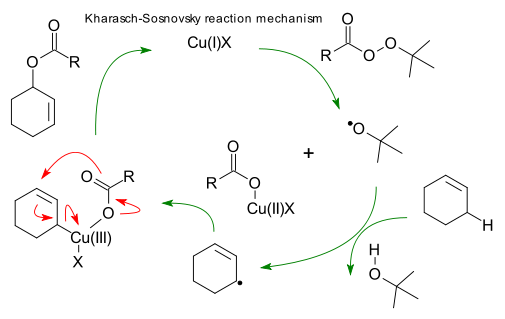
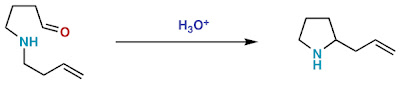
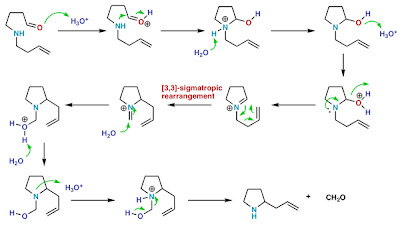
Stereochemistry of [n,m] Sigmatropic Rearrangements
Basically, [n,m] sigmatropic rearrangements can proceed through a chair or boat transition state. Only the chair transition state has been observed experimentally though both are suprafacial and are allowed in 4n+2 electron systems.
Chair and boat transition states in [n,m] sigmatropic rearrangements
Fig.1Chair

Fig.2Boat

Examples for sigmatropic rearrangements with a chair transition state
Fig.3Cope rearrangement

Fig.4[3,3] Sigmatropic
6 Electrons
Hückel aromatic
Supra-supra
 view animation,
view animation, 2D Animation of the Cope rearrangement
try copy paste of link below, do not miss out on a beautiful animation
http://www.chemgapedia.de/vsengine/supplement/Vlu/vsc/en/ch/2/vlu/pericyclische_reaktionen/pericyclisch_sigmatrop.vlu/Page/vsc/en/ch/2/oc/reaktionen/formale_systematik/pericyclische_reaktionen/sigmatrop/stereochemie_n_m.vscml/Fragment/0d47d3304bb08cfd9c68c1c3590965d2-19.html
Large substituents similar to their behavior in chair conformations of cyclohexane rings prefer an equatorial configuration in the transition state of [3,3] sigmatropic reactions. Heating S,S-3,4-dimethyl-1,5-hexadiene to approximately 200°C yields in 90% a product derived from a chair transition state with equatorial methyl groups. The product arising from a diaxial conformation is formed in only 10% yield. Obviously, the reaction does not proceed through the boat transition state.