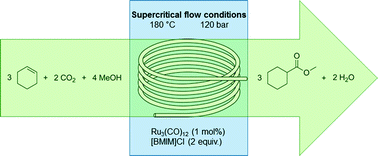
Highlights
HighlightsModification of BHT has a significant multivariate effect on antioxidant efficiency.
- BDE is the key to rational design and development of antioxidants.
- Antioxidant performance of BHT is mainly depending on 13 very crucial parameters.
- MPAO is a promising way to increase antioxidant and pharmacological activities.
Abstract
Hindered
phenols find a wide variety of applications across many different
industry sectors. Butylated hydroxytoluene (BHT) is a most commonly used
antioxidant recognized as safe for use in foods containing fats,
pharmaceuticals, petroleum products, rubber and oil industries. In the
past two decades, there has been growing interest in finding novel
antioxidants to meet the requirements of these industries. To accelerate
the antioxidant discovery process, researchers have designed and
synthesized a series of BHT derivatives targeting to improve its
antioxidant properties to be having a wide range of antioxidant
activities markedly enhanced radical scavenging ability and other
physical properties. Accordingly, some structure–activity relationships
and rational design strategies for antioxidants based on BHT structure
have been suggested and applied in practice. We have identified 14 very
sensitive parameters, which may play a major role on the antioxidant
performance of BHT. In this review, we attempt to summarize the current
knowledge on this topic, which is of significance in selecting and
designing novel antioxidants using a well-known antioxidant BHT as a
building-block molecule. Our strategy involved investigation on
understanding the chemistry behind the antioxidant activities of BHT,
whether through hydrogen or electron transfer mechanism to enable
promising anti-oxidant candidates to be synthesized.
Volume 101, 28 August 2015, Pages 295–312
Review article
Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review
- aNanotechnology & Catalysis Research Centre, (NANOCAT), University of Malaya, Block 3A, Institute of Postgraduate Studies Building, 50603 Kuala Lumpur, Malaysia
- bDepartment of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
- cDivision of Human Biology, Faculty of Medicine, International Medical University, 57000 Kuala Lumpur, Malaysia
- dDrug Design and Development Research Group, Department of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
- http://www.sciencedirect.com/science/article/pii/S022352341530101X
https://www.researchgate.net/publication/278050005_ChemInform_Abstract_Understanding_the_Chemistry_Behind_the_Antioxidant_Activities_of_Butylated_Hydroxytoluene_BHT_A_Review/figures








///////////Antioxidant, Butylated hydroxytoluene, Free radical, Reactive oxygen species, Phenol

















 .
.